

The designations cation or anion come from the early experiments with electricity which found that positively charged particles were attracted to the negative pole of a battery, the cathode, while negatively charged ones were attracted to the positive pole, the anode.įigure 4.5.1. To predict relative ionic sizes within an isoelectronic series.Īn ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or when additional electrons attach themselves to neutral atoms to form a negative one (anion).Periodic Table of Elements - Sodium - Na. If you need to cite this page, you can copy this text: This database focuses on the most common chemical compounds used in the home and industry. Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. Molar Mass Calculations and Javascript Calculator.Introduces stoichiometry and explains the differences between molarity, molality and normality. Related ResourcesĪnswers many questions regarding the structure of atoms. Common Chemical Compounds of Sodium ReferencesĪ list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.in nature sodium is fount in soda niter, cryolite, amphibole and zeolite. Other important forms of sodium are soda ash(Na2CO3), baking soda (NaHCO 3), Chili saltpeter (NaNO 3) which is sodium nitrate. Sodium chloride is common table salt which is important in animal nutrition. It never exists in nature, but is prepared by electrolysis of absolutely dry fused sodium chloride. It is the most abundant of the alkali metals. Sodium is the sixth most abundant element on earth comprising 2.6% of the earth's crust. Additional Notes: Sodium comes from the English word "soda" and from mideval Latin sodanum which means headache remedy.Also used in street lights, batteries, table salt (NaCl), and glass. Liquid sodium is sometimes used to cool nuclear reactors. Uses of Sodium: Used in medicine, agriculture and photography.Primary mining areas are Germany, Poland, Kenya USA. Approximate annual world wide production: sodium metal, 200,000 tons salt, 168,000,000 tons sodium carbonate, 29,000,000 tons. Sources of Sodium: Obtained by electrolysis of melted sodium chloride (salt), borax and cryolite.Name Origin: From soda (Na2CO3) Na from Latin natrium.Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances. Vapor Pressure = C Regulatory / Health.Enthalpy of Vaporization: 89.04 kJ/mole.Enthalpy of Atomization: 108.4 kJ/mole 25☌.Description: Soft silvery white alkali metal that reacts violently with water and oxidises rapidly when cut.Conductivity Electrical: 0.21 10 6/cm Ω.Coefficient of lineal thermal expansion/K -1: 70.6E -6.
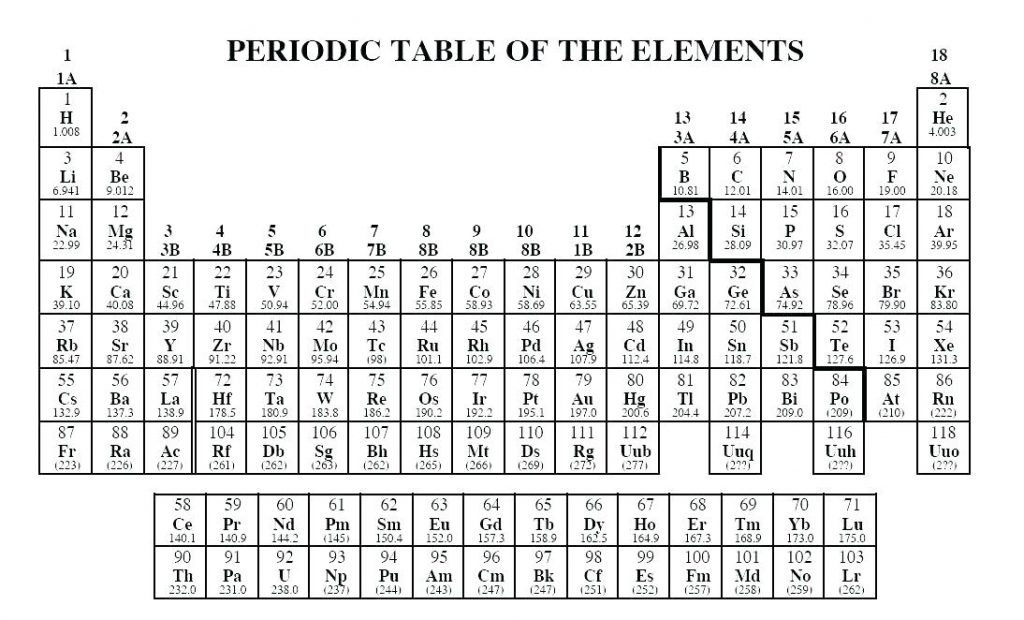
Valence Electron Potential (-eV): 14.1 Physical Properties of Sodium.Electronegativity: 0.93 (Pauling) 1.01 (Allrod Rochow).Electrochemical Equivalent: 0.85775g/amp-hr.Valence Electrons: 3s 1 Electron Dot Model.Number of Neutrons (most common/stable nuclide): 12.

Number of Electrons (with no charge): 11.
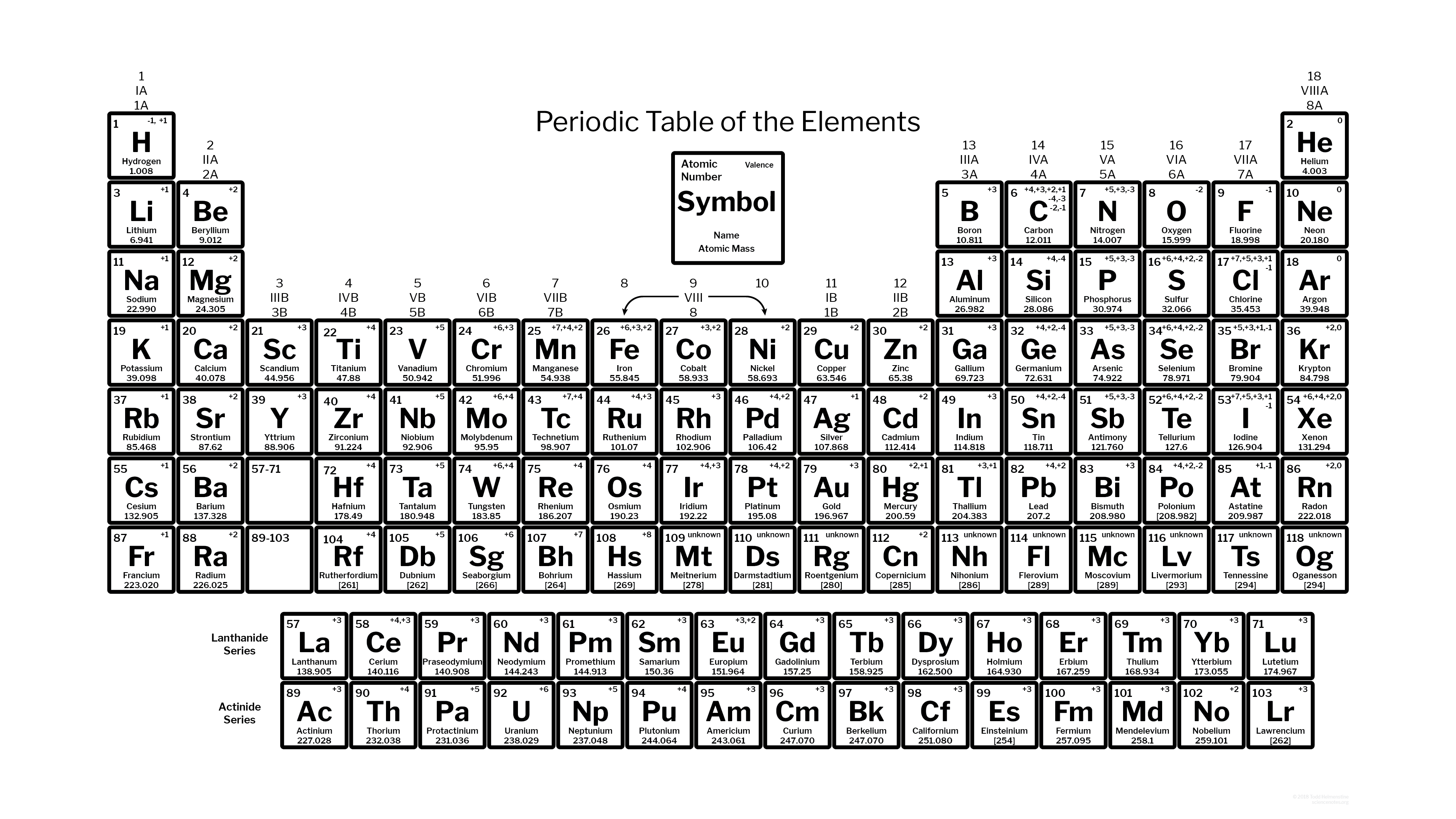


 0 kommentar(er)
0 kommentar(er)
